The magnetically soft materials are characterised as follows:
(i) They have high permeability.
(ii) The magnetic energy stored is not high.
(iii) They have negligible coercive force (due to which these are not suitable for making permanent magnets).
ADVERTISEMENTS:
(iv) They have low remanence.
The different magnetically soft materials are considered below:
1. Pure or Ingot Iron:
It has high permeability and increases with the stronger magnetising force.
It has a low resistivity (due to which production of eddy currents is encouraged).
ADVERTISEMENTS:
To reduce the eddy currents it (pure iron) is rolled into thin sheets, as a result of which is permeability decreases.
It undergoes a considerable improvement in its magnetic properties when traces of carbon, hydrogen and oxygen are reduced to the minimum limit.
In this material hysteresis loss is low.
It is unsuitable for alternating currents.
2. Cast Iron:
ADVERTISEMENTS:
It has carbon above 2.5 percent.
It is cheap but poor magnetic material.
It finds its use in the structure of electrical machinery and framework of D.C. machine.
3. Carbon Steels:
The steels may be low, medium and high carbon steels depending upon the percentage of carbon content. The low carbon steels in which percentage of carbon varies from 0.15 to 0.5 percent have properties comparable to those of pure iron.
ADVERTISEMENTS:
If the percentage of carbon is increased, it has following effects:
(i) Resistivity is increased.
(ii) Saturation temperature is lowered.
(iii) Mechanical strength is improved. 120,000
ADVERTISEMENTS:
(iv) Permeability is decreased.
(v) Coercive force and retentivity are increased.
It is due to these facts that high carbon 30 000 steel is used where mechanical strength is of primary importance as in the case of making rotors of turbo alternators. The low carbon steel however, is used in making parts or fabricated structures which carry 40,000 – either constant flux or no flux at all.
4. Silicon Steels:
A little addition of silicon to steel decreases permeability but reduces the hysteresis loss considerably. In addition, it reduces the eddy-current loss by increasing the electrical resistance.
Silicon improves magnetic properties.
The presence of silicon imparts hardness and raises the elastic limit of steel.
It is suitable for both a.c. and d.c. circuits and is used for the poles of motors and dynamos and cores of transformers.
The percentage of silicon varies for armature steel upto 0.5, for motor and dynamo steel from 2.5 to 3.5 and for transformer steel from 4 to 5.
Note:
Although silicon steel has less eddy current as well as hysteresis loss, dynamo steel is used for rotating machinery because of better mechanical properties, silicon steel being somewhat brittle; also dynamo steel is less expensive.
5. Manganese and Nickel Steels:
The addition of manganese and nickel to steel make it hard and almost non-magnetic. A high nickel manganese cast steel is used for making cable boxes, meter cases and end rings of turbo-alternators, for which non-ferrous metals are mechanically very weak and magnetic materials unsuitable.
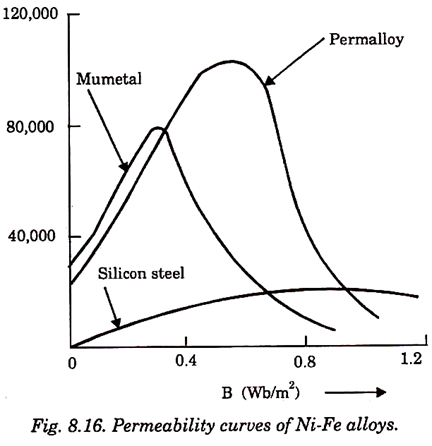
6. Permalloy:
It is a nickel iron alloy and contains: Ni-78.5%, Fe-21% and small quantities of Cr, Co, Cu, Mn etc.
Its permeability is 105.
Its resistivity is 17 μΩ cms approximately.
Hysteresis loss in this alloy is low at low magnetising fields.
Its magnetic properties are very sensitive and are affected even by normal handling.
If an addition of 4% of molybdenum and chromium is made to it, its resistivity increases from 17 to 60 μΩ cm without affecting the quality.
It is used as thin tape wrapped round the conductors of loaded submarine cables and as cases of loading coils placed at intervals in land long-distance telephone lines to make the electrical impulses distinct.
7. Mumetal:
It is also a nickel iron alloy.
Its percentage composition is- Ni—75.4%, Cu—4%, Cr—1.5% and the remainder iron.
Its magnetic properties are more stable than permalloy.
For optimum properties it is annealed in pure and dry hydrogen at 110°C for a number of hours and then slowly cooled.
Its saturation flux density is 1.3 Wb/m2.
Coercivity is less than one AT/m.
Its permeability is 100,000.
It is used for making cores of transformers where low coercivity is necessitated and also for the cores of intervalve audio frequency wireless transformers and in moving iron instruments.
8. Perminvar:
It is the name given to a series of cobalt-nickel-iron alloys.
Its composition is- 50% Ni, 25% Co, 25% Fe.
It has a constant permeability over a low flux-density range.
Its resistivity is about 18.5 micro ohm cm.
Hysteresis loss is low.
It is used in circuits where waveform distortion must be kept minimum such as armatures of motors, transformers cores etc.
Addition of small amounts of other elements to nickel iron alloys improve their properties in certain directions. Cobalt reduces hysteresis, manganese reduces coercivity and molybdenum increases the initial permeability and reduces the iron losses.
Since the saturation flux density of Ni-Fe alloys is low, they are not used in electrical machines but are mainly used in:
(i) Transformer cores,
(ii) Loading coils for telephone circuits,
(iii) Instrument transformers,
(iv) For the magnetic circuits of measuring instruments and
(v) For magnetic screens of electronic equipment.
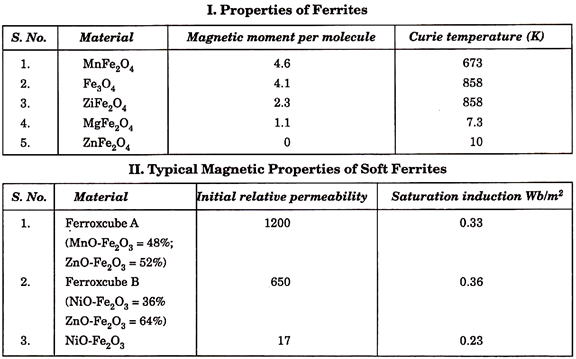
9. Ferrites:
They belong to class of soft magnetic materials and are so named because they are based on chemical compounds of the type, MeO. Fe2O3, where Me stands for a bivalent metal such as nickel, manganese, copper, magnesium, zinc, cadmium. A distinguishing feature of ferrites is their fairly constant permeability, and a narrow hysteresis loop, i.e., low losses in alternating magnetic fields. For this reason they are used in making cores for coils operating at elevated and high frequencies (in telephony and radio).
The manufacture of ferrites is carried out by ceramic techniques which consist in grinding the raw materials to a fine powder, making them up into mixtures suitable for shaping the requisite articles, and firing. Shaping is done either by pressing on steel moulds at a pressure of 1 to 3 tonnes/cm2 or by extrusion through a die of a mixture of the powdered constituents with an organic binding agent (polyvinyl alcohol, parafin wax).
Ferrites are fired at a temperature selected according to their composition and the shape and size of the articles. This temperature is generally between 1040°C to 1310°C and the firing is done for 4 to 6 hours with free access of air. Certain grades of ferrites, however, require firing in vacuum.
Shrinkage during firing is considerable and amounts to 10-20%. Therefore, it is difficult to produce articles to close, dimensional tolerances. Another disadvantage is that the fired articles cannot be machined, are hard to grind and are quite brittle.
The resistivity of ferrites is very much higher than that of ferromagnetic metals, since ferrites are chemical compounds and electrons in them are subject to restraint of valence forces.
General Electric and Magnetic Characteristics of Ferrites:
1. A permeability of several tens.
2. Extremely low dielectric loss.
3. A very high resistivity, generally more than 105 ohm-cm.
4. A microwave dielectric constant of the order of 10-12.
5. A Curie temperature which varies from 100°C to several hundred °C.
6. Mechanically hard, brittle and difficult to machine.
7. A saturation magnetisation which is appreciable, but noticeably smaller than that of ferromagnetic materials. The coercive force is low.
The synthesis of ferrites entails the following steps:
(i) Preparation of material;
(ii) Dry pressing;
(iii) Dying and removal of the binder;
(iv) Sintering;
(v) Annealing.
The raw materials are taken in proper proportions and well mixed in a ball milling machine which consists of a metallic cylinder and with free metallic balls.
The cylinder is rotated at a high speed and subsequently, due to random movement of metallic balls inside the cylinder the particle size is reduced and ultimately the ingredients are mixed properly.
The fine powder produced above is mixed with a suitable binder (polyvinyl alcohol is the most commonly used binder). The binder simply makes a paste of material and does not alter properties of the material.
The material is then given a desired shape, by the use of a suitable die through hydraulic pressing.
(iii) Drying and Removal of the Binder:
Drying operation is carried out to remove the binder material.
After dry pressing, the material (having acquired the shape of die) is then heated to temperature of 200-300°C (this range of temperature is suitable for only organic binder materials) thus removing the bulk of binder material from the material by evaporation.
Sintering is the process by which small particles of the materials are bonded together by solid state diffusion that is accomplished by firing at an elevated temperature. It results in the transformation of porous compact state into dense coherent product.
As the sintering progresses, the pores become smaller and more spherical in shape. The driving force for sintering is the reduction in total particle surface area; surface energies are larger in magnitude than grain boundary energies.
As the sintering process comes to an end, an equilibrium grain size is attained.
Annealing is a heat treatment process which is carried out to relieve stresses, increase softness, ductility and toughness and to produce a specified micro structure.
The sintered product, in our case, is annealed in an atmosphere of nitrogen.