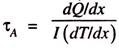Top 5 Methods of Refrigeration (Natural and Artificial Methods of Refrigeration)!
1. Natural Methods:
(a) By Using Ice or Snow:
The natural methods include the utilization of ice or snow produced naturally in cold climate. Ice melts at 0°C, so when it is placed in a space or system warmer than 0°C heat flows into ice and the space is cooled. The ice in turn melts into water by absorbing its latent heat at the rate of 335 kJ/kg. The river water, if it is quite cold, may also be used for cooling certain systems, but the removal of heat will not be as fast and satisfactory as in the case when ice or snow is used.
The natural methods of refrigeration were actually used in early days. With the present developments in science and technology and the subsequent rise in the standard of leaving the refrigeration requirements have become so large that the natural methods have become inadequate and therefore obsolete.
ADVERTISEMENTS:
When ice is to be used for refrigeration, winter ice can be stored by packing it in straw and dried weeds, for use in the summer months.
(b) Evaporative Cooling Process or Adiabatic Cooling Process:
In the old days, it was observed by the people that evaporation of water naturally causes cooling effect and hence they used to put their wine or other liquids into porus earthenware containers placed in the open atmosphere such as roof.
We have every day experience that water kept in an earthen pot becomes cooled because of the evaporation of water absorbing latent heat of evaporation from water in the pot and coming out through the pores. This effect is experienced very well in summer.
ADVERTISEMENTS:
Air flowing over the surface of water whose temperature is less than the incoming air, then the air is cooled because of the evaporation during which latent heat is taken from air and water sump.
Same effect is obtained when air is flowing across the spray of water. The cooling is taking place without exchanging the heat with the surrounding and this type of the cooling is called as Adiabatic cooling. Evaporation of sweat on our body thereby producing cooling effect is the best example of evaporative cooling.
2. Mechanical Refrigeration:
Artificial or mechanical methods of producing cold have been used since 1850. Methods of mechanical refrigeration include the use of non-condensable gases and condensable gases or vapours.
When non-condensable gases are used, refrigeration can be achieved by two methods such as:
ADVERTISEMENTS:
(a) Reversible or isentropic expansion of gases against the restraining force such as a piston-cylinder mechanism.
(b) Irreversible adiabatic expansion of gases from the high pressure to a low pressure through a very minute orifice or similar restriction.
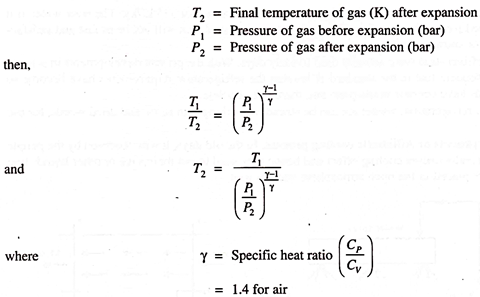
(a) Isentropic Expansion of Gases:
If we expand high-pressure air or gas in a piston-cylinder mechanism, then the temperature of gas decreases depending on the extend of expansion and the temperature of gas becomes less than atmosphere. This cold gas can be used to cool the object or the system. We know that if,
ADVERTISEMENTS:
T1 = Initial temperature of gas (K) before expansion
This method is very much used in air-craft refrigeration.
Air is compressed, cooled and then expanded so that refrigerating effect is achieved.
ADVERTISEMENTS:
Advantage of such a method is that the gas (air) is available free but the major disadvantage will be the lubrication difficulties for the piston cylinder mechanism.
(b) Refrigeration by Irreversible Adiabatic Expansion of Gases (Throttling) or Joule-Thomson Effect:
Throttling is an irreversible process in which a fluid, flowing across a restriction, undergoes a drop in total pressure. Such a process occurs in the flow through a porous plug, a partially closed valve, or a small orifice. Joule and Thomson performed the basic throttling experiments in the period 1852-62, and their experiments clarified the process and led to use of throttling as a method for determining certain properties of gaseous substances.
A steady stream of gas flows through a porous plug contained in a horizontal tube. This system is open, is thermally insulated (Q = 0) and does not exchange work with its environment (W=0). At sections 1 and 2 both the temperature and the pressure are measured. If the kinetic energy does not change significantly as the fluid passes through the porous plug, the steady, flow energy equation reduces to-
h1 = h2 …(1)
Hence, in an adiabatic throttling process the enthalpy remains constant. When a series of Joule-Thomson experiments is performed at the same initial temperature t1 and pressure p1 but with different downstream pressure, it is found that the temperature t2 changes. The results from these experiments can be plotted as a constant enthalpy curve on T-P plane.
If several different rates of flow are established at each condition of t1 and p1 a series of isenthalpic curves is obtained. The maximum point on each curve is called the inversion point and the locus of the inversion points is called the inversion curve. The slope of an isenthalpic curve is called the Joule-Thomson coefficient µh and is expressed by-
At the left side of an inversion point, µh is positive; at the right side of an inversion point, µh is negative; at the inversion point µh is zero. Since there is always a pressure drop in the throttling process, ΔP is always negative. Therefore when µh is positive, the temperature change is negative and throttling produces cooling.
Similarly, when is negative, the change is positive, and throttling produces a rise in temperature, even though the process is adiabatic. At any particular pressure, µh is positive only within a certain temperature range. These temperatures are called the upper and lower inversion points and are indicated in Fig. 36.4.
Between these two temperatures, throttling causes a drop in the temperature of the gas; outside of this temperature range, throttling results in a temperature rise. Above a certain pressure, throttling can cause only a cooling effect. This is point a in Fig. 36.4. Similarly, above a certain temperature throttling can cause only a heating effect. This is point b in Fig. 36.4.
In case of a perfect gas, enthalpy is a function of temperature alone, and therefore the temperature of the gas remains constant so that µh = 0. In case of a real gas the temperature generally does change with pressure and µh is a function of both temperature and pressure.
3. Steam-Jet Refrigeration:
This system is one of the methods employed by chemical agent machines for achieving mechanical refrigeration. Evidently water is used here as refrigerant.
Steam-Jet system is primarily used in installations where chilled water is required or the systems like cold storage where the temperatures required are more than 5°C as water starts changing its volume at 4°C and starts freezing at 0°C. The principle of flash cooling is employed in achieving the objective.
There are two important components in the system, (a) steam nozzle A and (b) the flash chamber. Also, there are two distinct circuits of fluids (i) steam flow circuit, (ii) water flow circuit in the system.
(i) Steam Flow Circuit:
High pressure steam is supplied to the nozzle entrance at A. Here it takes with it any vapour flashed in the flash chamber. The vapour is entrained or sucked by the suction created by the high velocity of steam flow in the nozzle.
Here the mixture expands in the converging portion upto the throat B. It is then compressed to a pressure say 5 cm Hg corresponding to 36°C temperature in the diverging diffuser. The compressed fluid passes on to the condenser. The condensate which consists of the flashed vapour and steam from supply source is pumped back to the boiler.
(ii) Water Flow Circuit:
The chilled water required is circulated from the flash chamber, by a pump on to the point of consumption, and the balance if any is returned back to the chamber as spray.
In the flash chamber low pressure of the order of 6 mm Hg is maintained due to the suction of vapour by steam flow at nozzle. The flash point is therefore low corresponding to saturation temperature at that pressure say 5°C.
The water is chilled due to absorption from it of the latent heat required for the evaporation of a certain fraction of water during flashing. The amount of water flashed in the chamber plus any other quantity of chilled water drawn from the chamber (say for drinking or other purposes) is made up by an equivalent amount of fresh supply through the spray.
It will be noted that for obtaining water at 5°C, with a condensing temperature say 35°C, the operating pressures are 6 mm Hg and 5 mm Hg respectively, which are extremely low values. The compression ratio of approximately 8 also approaches the limit of efficiency operation. Note, if temperatures below 0°C are required, antifreeze should be added to water.
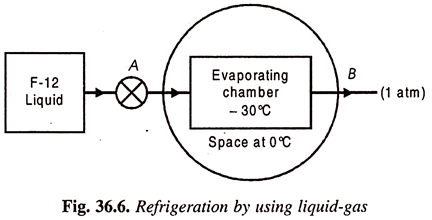
4. Refrigeration by using Liquid-Gases:
We know that a liquid can be vaporized at any desired temperature by changing its pressure. Further, heat is required to be added to the liquid during vaporization when the liquid phase changes to the gaseous phase. Accordingly, a vaporizing liquid can to produce refrigeration at any temperature. For instance, at a pressure of about 1 atm, ammonia may be made to boil at -33°C or at a pressure of about 5 atm. Freon -22 boils at 0°C.
Hence in an arrangement in which a system containing some refrigerant in liquid form at a certain pressure is exposed to another system at temperature greater than refrigerating temperature.
A simple scheme of such an arrangement is shown in Fig. 36.5. Liquid Freon F-12 is supplied to the evaporating chamber through a valve at a pressure of about 1 atm. Since the boiling temperature of F-12 at this pressure is – 30°C, heat flows from the surround space at 0°C and makes the F-12 boil. Thus the space will be cooled as long as there is a supply of liquid F-12 to the evaporating chamber.
The arrangement of Fig. 36.6, however, suffers from two important drawbacks:
(a) The cost of replacing Freon-12 (an expensive refrigerant) will be prohibitive as the evaporated vapour leaks out to the atmosphere.
(b) If the system were to use some other refrigerant like ammonia, it may become a hazard to life due to its discharge to atmosphere since it is a highly toxic and irritating fluid.
To eliminate these drawbacks, it will be necessary to let the refrigerant fluid work in a closed loop and be used again and again.
5. Thermo-Electric Refrigeration:
Thermoelectric effects refer to phenomenon involving the interchange of heat and electrical energy. One familiar example is the heating effect due to the flow of an electric current in a resistor. The electric power dissipation depends on the current flow I and the potential V according to the equation P = VI or P = VI = I2R. This type of energy conversion is irreversible, although some thermoelectric effects are reversible.
There are three thermoelectric effects.
They are:
(i) The See-beck effect,
(ii) Peltier effect, and
(iii) Thomson effect.
(i) The See-beck Effect:
In 1833 See-beck held the junctions of two dissimilar electrical conductors at different temperatures and noted that an open-circuit voltage was generated that was proportional to the difference in temperature between the two junctions. A familiar example which illustrates the See-beck effect is the thermocouple. The heat supplied at one of the junctions is converted into electrical energy, which is then measured.
(ii) The Peltier Effect:
In the Peltier effect, which is the inverse of the See-beck effect, an electric current is sent through a junction of two dissimilar materials A and B. and this results in either a heating or cooling of the junction. The rate of heating or cooling is proportional to the current flow, and is given by the equation
Q = πABI
where the coefficient πAB is a function of temperature called the Peltier coefficient. The subscript AB indicates that the Peltier coefficient also depends on the two materials A and B. A typical unit of πAB is the volt.
If current flows in the direction of the potential drop characteristic of the junction, then the temperature of the junction decreases, and a refrigerating effect occurs in the space surrounding the junction. If current flows in the direction of the junction’s potential rise, the temperature of the junction increases and heat must be transferred from the junction to maintain constant temperature at the junction.
(iii) The Thomson Effect:
When art electric current flows through a homogeneous material, heat is exchanged between the environment and the material, and the rate of transfer of heat depends on both the quantity of current that flows through the material and the temperature gradient that exists in the material. The Thomson coefficient τ is defined as-
where dQ/dx is the rate of heat interaction per unit length of the conductor and dT/dx is the temperature gradient along the length of the conductor. Like the See-beck and Peltier effects, the Thomson effect is reversible; on the other hand, it involves only a single substance.
The See-beck, Peltier and Thomson effects occur in both metals and semiconductors, but these effects are more pronounced in semiconductors.
If See-beck would probably have tried reversing the direction of flow of electrons in the thermoelectric circuit (by externally applying a potential difference in the reverse direction), he would have found a refrigerating effect. But this very important effect (refrigeration) was discovered by Mr. Jean Charles Athanase Peltier, in 1834. He observed during his experiments that when a small current was passed through the junction of two dissimilar wires, the junction was cooled.
Peltier effect is the basis for thermoelectric refrigeration.
Thermoelectric refrigerators cannot compete with the vapour compression refrigeration systems because of its low coefficient of performance, small refrigerating effect. They are preferred in some specific applications because of their small size, simplicity, quietness and reliability.
Mention may be made here of certain non-conventional refrigeration system which are used for specific applications only.
Non-conventional refrigeration systems are:
(i) Magnetic refrigeration system
(ii) Vortex-tube system
(iii) Pulse jet refrigeration system
(iv) Ultrasound refrigeration system