In this article we will discuss about the allotropy of metals.
Many of the metallic elements (even compounds such as silica, etc.) exist in more than one crystalline form (but one at a time) depending upon the external conditions of temperature or pressure, or both. This phenomenon is called polymorphism or allotropy. Multiple crystal structures of the same composition are generally called polymorphs.
The term allotropy is normally reserved for this behaviour in pure elements, while polymorphism is a more general term. The different phases are called allotropes. This change can also occur in some elements when subjected to unusual thermal or mechanical treatments.
Under normal conditions of atmospheric pressure, each allotropic form of a metal with a definite crystal structure exists at equilibrium over a range of temperatures, and then changes to another crystal form at a critical temperature.
ADVERTISEMENTS:
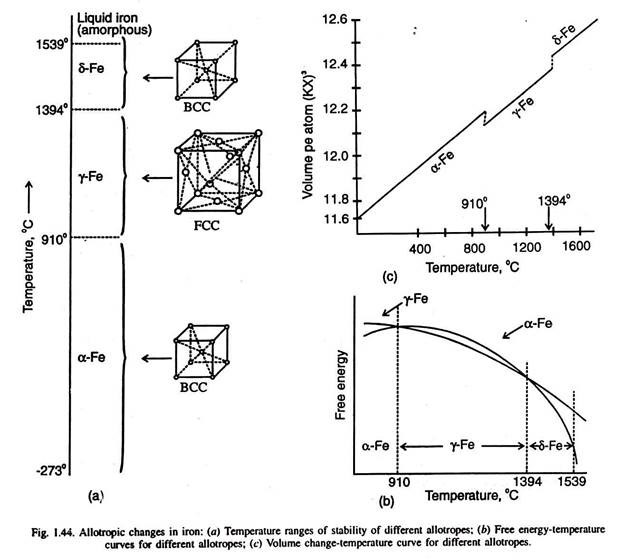
Normally, the allotrope stable at the lowest temperature is called by Greek- letter- α (alpha) preceding the chemical symbol of the metal, such as α -Fe. The next higher temperature modification is called β, then γ and at still higher temperatures as δ (delta). Fig. 1.44 illustrates the allotropic changes in iron with changes in temperature at atmospheric pressure. Below 910°C, iron has BCC crystal structure, and is called α -iron. But above 910°C, iron is called γ -iron with FCC crystal structure.
At 1394°C, γ -iron changes to δ-iron (BCC structure), the second allotropic change. Fig. 1.44 (b) illustrates that as the free energy of a phase becomes less at a temperature, it becomes stable and the earlier phase transforms to this stable phase. Almost all properties change when a metal changes from one allotrope to another. Fig. 1.44 (c) illustrates the change in volume with the change in allotropic forms (as temperature is varied).
A polymorphic or allotropic change is a phase change, just like a change from the liquid to the solid phase. Thus, as happens in the freezing process, here too, two phenomena are observed- (a) supercooling, (b) evolution of heat of reaction. Both these effects are also observed with phase transformations in the solid state, i.e., in polymorphic changes.
ADVERTISEMENTS:
Supercooling is the delay of a phase change to a temperature below the equilibrium temperature. The liquids supercool a few degrees before freezing occurs, but solids can be supercooled too much greater extent before the new phase forms. The new crystals formed as a result of allotropic transformation are of different size and shape.
For example, if a metal is heated to a temperature slightly more than the critical temperature of polymorphic change, such as α-iron is heated to a temperature slightly more than 910°C, fine grains of g-iron are formed. Thus, phase transformation helps in refining the grains, if we had earlier coarse grains.
Allotropic transformation in metals are studied very often by thermal analysis method, which consists in detecting departures from the normal cooling (or heating) curve of a substance due to evolution (or absorption) of heat of reaction during phase transformation. Thus, the departure from the normal cooling curve indicates the phase transformation occurring.
Allotropic changes take place with abrupt changes in all properties of metals and alloys like specific volume, thermal conductivity, electrical conductivity, magnetic properties; mechanical and chemical properties, etc. Table 1.7 shows some metals having different crystal structures due to polymorphic change.
Problem:
Titanium undergoes a change in phase BCC to HCP at 880° C on cooling, Calculate the % volume change. Given lattice parameters,
aBCC = 3.32 A,
and aHCP = 2.956 A and c = 4.683 A.
ADVERTISEMENTS:
Solution:
Our base is unit cells of both crystal structures. There are 2 atoms per unit cell in BCC structure but are 6 atoms per unit cell in HCP structure. As the number of atoms remains the same after the phase transformation, therefore 3 unit cells of BCC give one unit cell of HCP. Let us find volume of one unit cell of HCP and three unit cells of BCC and find the difference in volume.
Volume of 3 unit ceils of BCC = 3(3.32 A)3
= 109.8 A3
ADVERTISEMENTS:
Volume of a unit cell of HCP- There are six equilateral triangles in the basal plane. Thus, volume of one unit cell of HCP
Thus, there is contraction when titanium (BCC) transforms to titanium (HCP) on cooling. Contraction also takes place when α-Fe transforms to γ-Fe on heating as illustrated in Fig. 1.29 (c), when the sudden decrease of volume takes place at 910°C.
Polymorphic transformations have also been induced by applying high pressures. The transformations with increasing pressure usually yield a more dense crystal structure (close-packed), which is also according to the Le Chatelier’s principle.
Pure iron at one atmospheric pressure, as we saw, exists as alpha (BCC) and gamma (FCC) iron, but changes to a new crystalline phase HCP (ԑ) at pressures above 100 k bar with a = 2.95 A°, c = 3.93 A°, and c/a = 1.61.
Polymorphic change can be categorised in two classes based on the reversibility of the change as:
(i) Enantiotropy (Greek Word-Opposite Change):
The polymorphs are said to be enantiotropic, when each has a definite range of stability, and one form changes to another, reversibly, at definite transition points defined by the pressure or and temperature as happens in case of iron. This type of change is common. For example- Fe, Ti etc.
(ii) Monotropy (Greek Word-One Change):
Monotropy is the state when one polymorph is stable, while the other is metastable over the whole range of existence. Here only the metastable form can change to the stable form. For example, phosphorous.
The kinetics of polymorphic changes varies widely from infinity to zero. Advantages are derived if the metastable form can continue to exist beyond the stability range, particularly if this form has more desirable properties.
A high temperature phase austenite can be made to exist (in alloy steels) at room temperature by suddenly cooling to room temperature, where it can exist almost permanently for all practical purposes, as the kinetics of its transformation to more stable form is very slow.